In preventing nuclear collapse in cells, scientists inch closer to a treatment for a set of rare diseases
University of Minnesota researchers have discovered a potential target for the research and development of future therapies to mitigate the symptoms of a series of genetic disorders called laminopathies. Laminopathies cause a wide variety of conditions in humans, from skeletal muscular dystrophy and neuropathy to premature aging.
In a study published in the Proceedings of the National Academy of Sciences (PNAS), researchers set out to better understand how mutations in proteins that associate with the nuclear envelope — which encases each cell’s nucleus — cause laminopathies.
“One of the proteins that we are interested in is torsinA. When this protein is not working properly in humans, it can cause a disease called early-onset dystonia, which has no cure and causes uncontrollable muscle contractions,” said study lead Gabriela Huelgas Morales, a postdoctoral associate in the College of Biological Sciences. “We wanted to find out how this protein works and what could be done to help cells maintain their normal functions.”
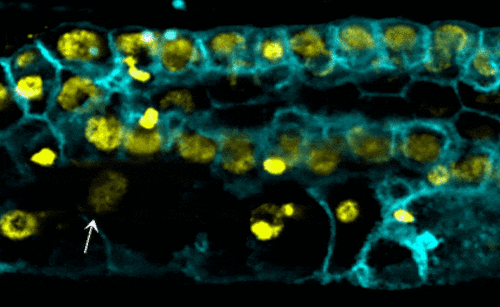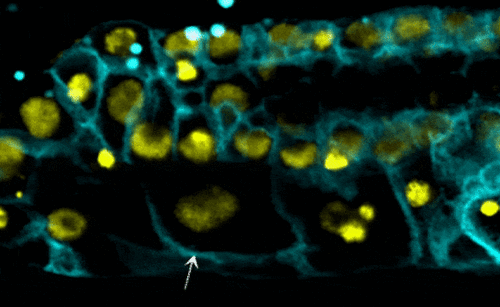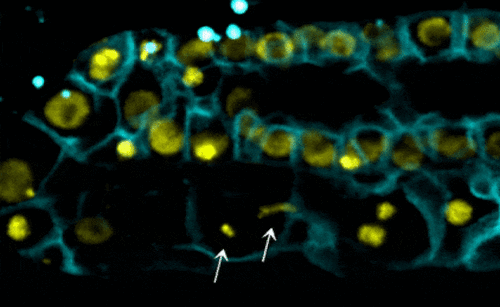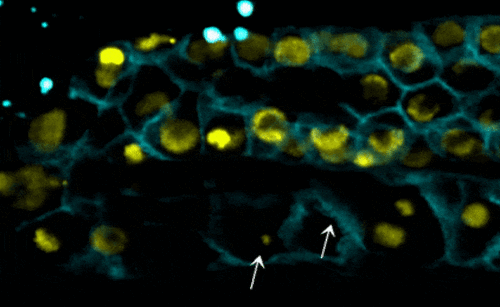
Scientists examined the interactions between proteins within the nuclear envelope of Caenorhabditis elegans, a worm used for some types of medical research. In their research, they found that torsinA interacts with the lamin protein, which provides structure inside the nucleus, and with components of the LINC complex, which transmits mechanical signals and forces across the nuclear envelope. When the lamin protein is mutated in the worms, the life span, development of reproductive organs, and the viability of off-spring are negatively impacted.
Through their work, researchers found:
- worms with the mutated lamin have frail nuclei that are prone to rapid collapse — a new phenomenon with relevance to laminopathies (see video);
- decreasing the function of torsinA in worms with mutated lamin proteins — in this case, by removing one copy of the torsinA gene — prevented nuclear collapse and extended the worms’ lifespan; and
- that, ultimately, torsinA promotes the function of the LINC complex.
“While there is much more research to be done, these findings point to possible future opportunities to create therapies to target the torsinA–LINC complex nexus,” said study co-author David Greenstein, a professor and the associate dean of research in the College of Biological Sciences. “In doing so, cells can maintain their structure and potentially alleviate symptoms caused by laminopathy diseases.”
Funding for the study was supported by the U.S. National Institutes of Health (GM57173 and NS095109) and the Dystonia Medical Research Foundation’s Barbara Oliver Memorial Dystonia Research Award.
- Categories:
- Science and Technology





