A team of energy researchers from the University of Minnesota and University of Massachusetts Amherst has discovered that molecular motion can be predicted with high accuracy when confining molecules in small nanocages. Their theoretical method is suitable for screening millions of possible nanomaterials and could improve production of fuels and chemicals.
The research is published online in ACS Central Science, a leading open-access journal of the American Chemical Society.
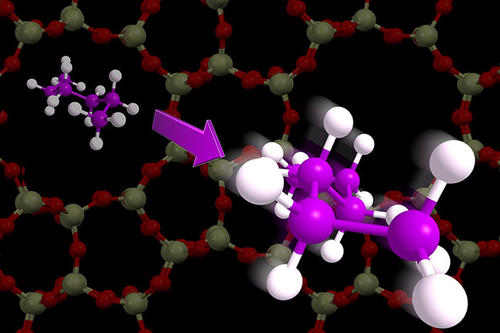
Molecules in the air are free to move, vibrate and tumble, but confine them in small nanotubes or cavities and they lose a lot of motion. The total loss in motion has big implications for the ability to capture CO2 from the air, convert biomass molecules into biofuels, or to separate natural gas, all of which use nanomaterials with small tubes and pores.
Researchers from the Catalysis Center for Energy Innovation headquartered at the University of Delaware arrived at their breakthrough when thinking about squeezing molecules into tight spaces. In the air, molecules can move up, down, and into space (three dimensions), but in a nanotube it was not clear if molecules can only move in one direction (through the tube) or two directions (on the surface of the tube). Similarly, molecules can rotate and spin in three ways, but the tube edges can prevent some or all of this motion. The amount of lost rotation was the unknown quantity.
“Our approach was to separate out molecular tumbling and rotating from movement in position,” said Omar Abdelrahman, a co-author of the study who is a University of Massachusetts Amherst chemical engineering assistant professor and Catalysis Center for Energy Innovation researcher. “We discovered that all molecules when put into nano-cages lose the same amount of movement in position, but the amount of rotating and spinning depended highly on the structure of the nano-cage”.
The team connected molecular motion to the quantity of entropy, which combines all aspects of molecular motion into a single number. Molecules lose different amounts of entropy when they access the inside of nanoporous spaces, but it has not been clear how the structure of those nanospaces impacted the change in motion and loss in entropy.
“It might sound esoteric, but the entropy changes of molecules due to limitations of rotation and movement in position within nanopores decides whether nanomaterials will work for thousands of energy and separation technologies,” said Paul Dauenhauer, a co-author of the study who is a University of Minnesota chemical engineering and materials science associate professor and Catalysis Center for Energy Innovation researcher.
“If we can predict molecular motion and entropy of molecules, then we can quickly determine whether advanced nanomaterials will solve our most pressing energy challenges,” Dauenhauer added.
The ability to predict entropy and molecular motion is connected to the recent nanotechnology boom. In the past decade, research in nanomaterials has developed millions of new technologies that can grab, separate and react hydrocarbons from natural gas and biomass. However, each of these thousands of nanomaterials has a different size and shape, and it has been too expensive and time consuming to test these advanced nanomaterials one by one.
“This discovery really opens up the door to predicting which nanomaterials will be the breakthrough of the future,” said Dionisios Vlachos, the Catalysis Center for Energy Innovation director and University of Delaware professor. “We have invented more materials on the computer than we can ever test, and now we can rapidly determine on the computer if these will work for our energy and separation needs.”
The focus on predicting molecular motion in nanomaterials builds on the Catalysis Center for Energy Innovation’s focus on design of catalysts for converting biomass-derived hydrocarbons into biofuels and biochemicals. The team recently discovered a new class of nanomaterials called “SPP” or “self-pillared pentasils,” which are zeolite nanomaterials for reacting and separating hydrocarbons. SPP and other nanostructures have also been the key materials in discovering chemical processes to make renewable plastic for soda bottles and renewable rubber for automobile tires.
The discovery of an equation for predicting molecular motion in nanomaterials is part of a larger mission of the Catalysis Center for Energy Innovation, a U.S. Department of Energy–Energy Frontier Research Center, led by the University of Delaware. Initiated in 2009, the Catalysis Center for Energy Innovation has focused on transformational catalytic technology to produce renewable chemicals and biofuels from lignocellulosic (non-food) biomass.
This research was funded by the Catalysis Center for Energy Innovation as part of the U.S. Department of Energy–Energy Frontiers Research Program. The Catalysis Center for Energy Innovation is led by University of Delaware Professor Dionisios Vlachos and co-directed by University of Minnesota Associate Professor Paul Dauenhauer.
To read the full research study entitled “A Universal Descriptor for the Entropy of Adsorbed Molecules in Confined Spaces,” visit the ACS Central Science website.
- Categories:
- Science and Technology





